Technical Details
Handling Instructions
Ambient temperature. Please refer to Technote Shipment of CellGenix® GMP Media at Ambient Temperatures
Store at +2°C to +8°C. Light protection recommended.
Primary Packaging Details
Medium Bottle
- Material of bottle: Polyethylene Terephthalate (PET), classified USP Class VI
- Material of bottle top: High-Density Polyethylene (HDPE), classified USP Class VI
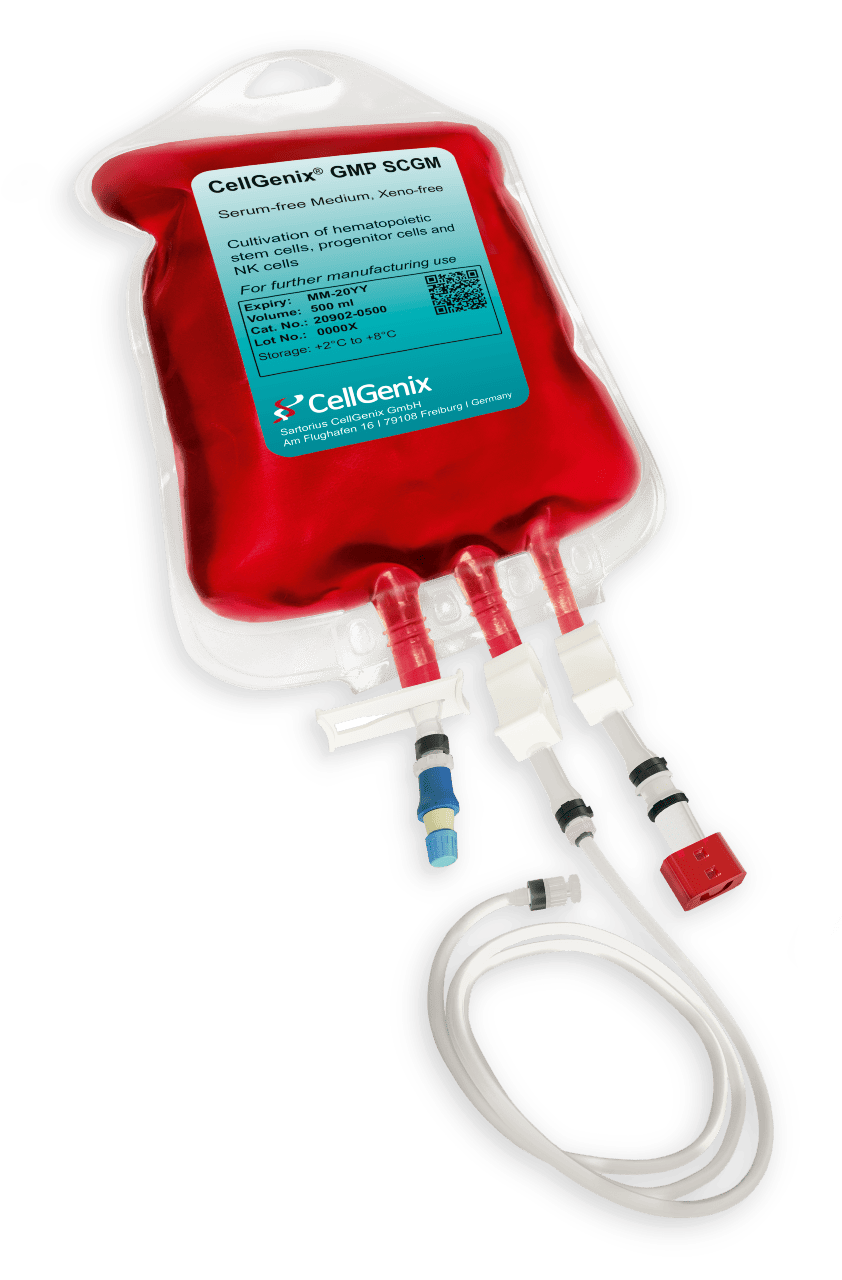
Medium Bag – Flexboy®
- Material of bag chamber: Ethylene-Vinyl Acetate (EVA) with a core layer of Ethyl Vinyl Alcohol (EVOH) as gas barrier, classified USP Class VI
- Material of bag tubing: Ethylene-Vinyl Acetate (EVA), Silicone, PVC (DEHP-free, suitable for sterile welding), classified USP Class VI
- Ports: 1x Male Luer Lock Port 1/8″, 1x Female Luer Lock Port 3/16″ with 1x Clave® Connector, 1x filling port (exclusively used for filling the media into the bag during the manufacturing process)